Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jan 28, 2026

The Anatomy of Coil Gunk
For e-liquid formulators and discerning consumers alike, the holy grail of vaping is a clean, flavorful, and consistent aerosol. We chase the perfect “puff”—an instantaneous transition from liquid to vapor that delivers authentic taste without residue. Yet, the industry is plagued by recurring issues: burnt hits, muted flavor profiles after a few milliliters, and the dreaded black crust that accumulates on heating coils, affectionately known as “coil gunk.”
Often, these problems are blamed on sweeteners or “bad batches” of flavor concentrate. While these play a role, the root cause of poor vaporization performance is frequently far more fundamental. It is a matter of physics and chemistry, boiled down to a single, crucial characteristic: Molecular Weight (MW).
At CUIGUAI Flavor, we don’t just mix flavors; we engineer them at a molecular level. We understand that an e-liquid is a complex matrix composed of carriers (Propylene Glycol and Vegetable Glycerin), nicotine, and hundreds of aroma compounds. For this matrix to function correctly in a vaping device, every component must cooperate with the thermodynamics of vaporization.
When flavor molecules are too heavy, they refuse to cooperate.
This article will provide a deep, technically detailed examination of why heavy molecules fail in vaping applications. We will explore the relationship between molecular weight, intermolecular forces, and volatility, explaining exactly how heavy compounds sabotage the vaping experience from the inside out.
Before delving into molecular weight, it is vital to establish precisely what happens when an e-cigarette is fired. The terminology is often used loosely, but scientifically, these distinctions matter.
Vaping devices are designed as electronic nicotine delivery systems (ENDS) that utilize heat to create an inhalable mist. The primary mechanism desired is vaporization. This is a phase transition where a substance turns from a liquid state into a gaseous state. In vaping, this generally occurs through boiling—supplying heat energy to the liquid until its vapor pressure equals the surrounding atmospheric pressure.
Ideally, the PG, VG, and flavor compounds all reach their respective boiling points efficiently, transitioning into gas.
Technically, what a user inhales is not a pure gas, but an aerosol. As the vaporized gas leaves the hot coil and encounters cooler air in the atomizer chamber and chimney, it rapidly condenses back into tiny liquid droplets suspended in the air. This dense fog of droplets is what we call “vapor.”
This is crucial: Vaping is not smoking. Smoking relies on combustion—burning organic material in the presence of oxygen at temperatures exceeding 600°C (1112°F) to create smoke containing thousands of new chemicals.
Vaping devices are designed to operate much cooler, typically between 180°C and 250°C (356°F – 482°F). The goal is to heat the liquid enough to turn it into gas without breaking the chemical bonds of the molecules.
If an e-liquid component requires a temperature of 350°C to vaporize, but the device only supplies 250°C, that component will not turn into gas. Instead, it sits on the coil, absorbing heat until it undergoes pyrolysis—thermal decomposition in the absence of oxygen. The molecule breaks apart, burns, and turns into carbon char. This is the origin of the “burnt hit” and coil gunk. Heavy molecules are the primary culprits in this scenario.
At its most basic, molecular weight (often referred to as molar mass in chemistry) is the mass of a given molecule. It is typically measured in Daltons (Da) or grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a chemical formula.
Consider two different components commonly found in the vaping world:
To visualize why this matters in vaporization, imagine trying to throw objects into the air.
Think of heat energy from the coil as the force of your throwing arm.
A light molecule (like water or a simple fruit ester) is a tennis ball. With minimal effort, you can throw it high into the air (vaporize it).
A medium molecule (like PG or VG) is a baseball. It requires more effort, but it’s manageable.
A very heavy molecule (like a lipid or wax) is a bowling ball. No matter how hard you throw (how much heat you apply), that bowling ball is barely getting off the ground. It will likely just sit there, absorbing the energy until it eventually catches fire or crumbles.
In the microscopic world of the atomizer, heavy molecules are bowling balls that refuse to fly.
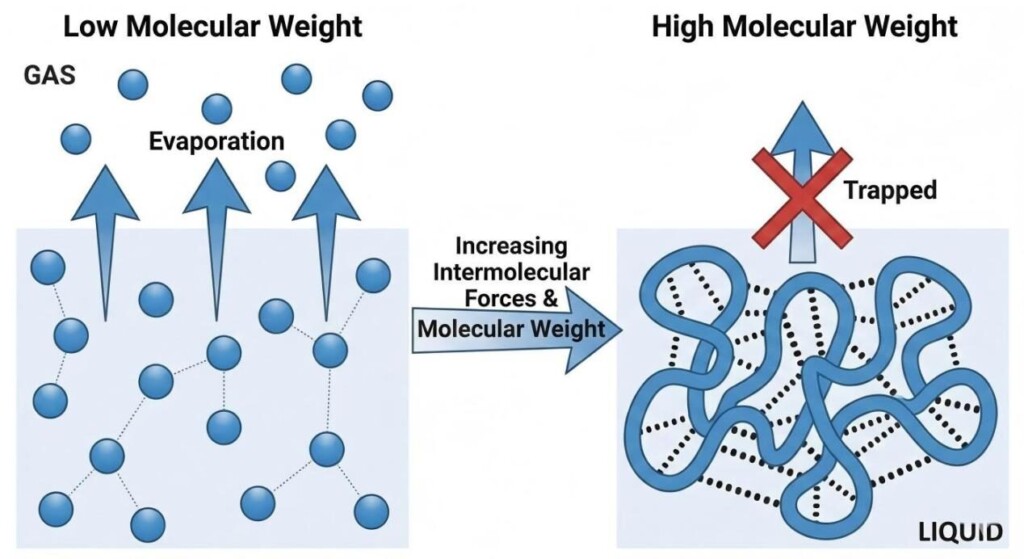
Why does weight make such a difference? It isn’t just gravity. The relationship between molecular weight and the ability to vape stems from volatility and intermolecular forces (IMFs).
Volatility is the tendency of a substance to vaporize. A highly volatile substance (like alcohol or gasoline) evaporates quickly at room temperature. A low-volatility substance (like motor oil) does not. In e-liquid formulation, we need compounds with relatively high volatility to match the operating temperatures of vaping devices.
Heavier molecules generally have lower volatility due to stronger intermolecular forces.
Molecules in a liquid are held together by attractive forces. To turn that liquid into a gas, you must add enough kinetic energy (heat) to overcome these forces, allowing the molecules to break free from their neighbors and escape into the vapor phase.
There are several types of IMFs, but two are critical here:
Because heavy molecules experience stronger IMFs, they require substantially more energy (higher temperatures) to reach their boiling point.
If we look at general chemistry principles, we see a clear trend: as the carbon chain length of a molecule increases (adding weight), the boiling point rises. According to educational resources like Chemistry LibreTexts, the boiling point of organic compounds increases with molecular weight due to the corresponding increase in the strength of intermolecular forces, requiring more energy to separate the molecules.
When a flavor compound is too heavy, its required boiling point might exceed the safe operating limits of the vaping device (e.g., >300°C). The device cannot supply the energy “kick” needed to launch that heavy molecule into vapor.
Molecular Weight & Vaporization Forces
E-liquid is a solution. The behavior of the overall liquid is dictated by the interaction between the solvent (the carrier base) and the solutes (flavorings and nicotine).
The base of e-liquid is chosen for its relatively low molecular weight and appropriate boiling points:
VG is heavier and “stickier” (more hydrogen bonding) than PG, which is why high-VG liquids are thicker and require slightly more power to vaporize efficiently. However, both fall within the acceptable range for standard vaping hardware.
When you introduce a heavy flavor molecule—for example, a complex resin found in a natural tobacco extract or a lipid used in an improper formulation—it is dissolved or suspended in this PG/VG matrix.
According to Raoult’s Law and the principles of colligative properties, adding a non-volatile (heavy) solute to a solvent lowers the overall vapor pressure of the solution and elevates its boiling point. This means the very presence of heavy flavor molecules makes the entire e-liquid harder to vaporize, requiring more power from the battery and more heat at the coil.
What happens physically on the atomizer coil when an e-liquid containing heavy molecules is vaped? The results are detrimental to both the user experience and the hardware.
Vaporization in an e-cigarette is a violent, rapid process. When the coil heats up, the components of the liquid do not vaporize simultaneously. The lightest, most volatile compounds (PG, certain fruit esters, alcohols) flash into vapor first.
If heavy molecules are present, they “lag” behind. As the lighter carrier liquid evaporates, the remaining liquid on the wick becomes increasingly concentrated with the heavy, non-volatile sludge. This is a microscopic form of fractional distillation occurring in the cotton wick.
Over a few milliliters of vaping, the liquid touching the coil is no longer the balanced e-liquid you started with; it is a concentrated goo of heavy flavorants.
This concentrated heavy goo coats the heating wire. Heavy organic molecules are generally poor conductors of heat. This coating acts as thermal insulation.
The coil now has to work harder to push heat through this layer to reach fresh e-liquid. The user experiences this as a “weak hit” and intuitively turns up the wattage. This only exacerbates the problem by heating the insulating layer even hotter without vaporizing it. The result is muted flavor because the volatile aroma compounds are trapped behind a wall of heavy sludge.
This is the terminal failure mode. As the user increases power, or as the heavy layer sits on the hot metal repeatedly, the temperature of this sludge exceeds its thermal stability limit.
Since the molecules are too heavy to fly away as gas, they sit there and cook. Their chemical bonds break down thermally (pyrolysis). Hydrogen and oxygen atoms may escape, leaving behind a carbon-rich residue. This is polymerization and carbonization in action.
This residue is “coil gunk.” It is often acrid, tasting of burnt sugar or charred carbon. Once a coil is heavily gunked, it cannot be recovered. The carbon layer continues to burn with every puff, ruining the flavor of even fresh e-liquid added to the tank. Research into e-cigarette aerosol chemistry has repeatedly shown that thermal degradation of e-liquid components leads to the formation of harmful carbonyls (like formaldehyde and acrolein), and this degradation is significantly accelerated when non-volatile compounds accumulate on the heating element.

Microscopic Wicking Contamination
Not all flavorings are created equal. Some are inherently unsuitable for vaporization due to their molecular weight and composition.
These are the absolute worst offenders. Triglycerides (vegetable oils), waxes (from plant cuticles in natural extracts), and long-chain fatty acids have extremely high molecular weights (often >300-500 g/mol).
They do not vaporize under normal vaping conditions. They immediately deposit on the coil and burn. More dangerously, if inhaled as aerosol droplets, heavy oils can accumulate in the lungs, leading to severe respiratory issues, as tragically seen in the EVALI crisis linked to Vitamin E Acetate (a heavy, oily thickening agent). The Centers for Disease Control and Prevention (CDC) identified Vitamin E Acetate as a primary cause of EVALI, highlighting the severe danger of inhaling heavy, oily compounds that the lungs cannot process.
While “natural” sounds appealing, crude natural extracts (like unprocessed tobacco absolutes or certain vanilla oleoresins) contain a full spectrum of plant compounds. Many of these, such as plant resins, lignins, and complex polysaccharides, are massive molecules totally unsuitable for vaping. They guarantee rapid coil destruction.
Sucralose is often cited as a coil-gunker. Its molecular weight (397.6 g/mol) is high, but its primary failure mode is thermal instability. It caramelizes and burns at relatively low temperatures, forming a stubborn carbon crust. While related to weight, it’s more about its chemical fragility under heat.
At CUIGUAI Flavor, we understand that creating a great e-liquid flavor is not just about matching a taste profile; it’s about ensuring that profile can survive the vaporization process intact.
We employ “volatility engineering” in our flavor design.
We prioritize aroma compounds with molecular weights and boiling points compatible with PG/VG vaporization thresholds. We utilize esters, aldehydes, ketones, and alcohols that are known to vaporize cleanly.
In perfumery and flavoring, “base notes” are usually heavier molecules that last longer. In vaping, we must be extremely judicious with base notes. We use only those heavy enough to provide depth and lingering flavor, but light enough to eventually vaporize off the coil rather than accumulate as permanent gunk.
By rigorously analyzing the molecular weight profiles of our raw materials using gas chromatography-mass spectrometry (GC-MS), we ensure that our finished flavor concentrates are free from the heavy “bowling balls” that ruin the vaping experience.
Selective Molecular Filtration
The difference between a mediocre e-liquid that destroys coils in a day and a premium product that offers consistent flavor for weeks is often invisible to the naked eye. It lies in the molecular weight of the flavor compounds chosen by the formulator.
Heavy molecules are fundamentally incompatible with the physics of vaping. They resist aerosolization due to strong intermolecular forces, they accumulate on heating elements through fractional distillation, and they thermally degrade into insulating, acrid coil gunk.
For e-liquid manufacturers, understanding this is not optional—it is essential to product success and consumer safety.
By partnering with a flavor house that understands the thermodynamics of vaporization and prioritizes volatility engineering, you ensure your final product delivers the clean, potent, and reliable experience that today’s educated consumers demand. Don’t let heavy molecules weigh down your brand’s reputation.
Are you struggling with coil longevity issues, muted flavor profiles, or inconsistent vaporization in your current e-liquid formulations?
Let’s talk science. CUIGUAI Flavor specializes in optimizing flavor performance for the unique demands of vaping hardware. We invite e-liquid manufacturers and formulators to connect with our technical team.
Request a Technical Consultation or Free Specialized Sample Kit:
We can analyze your current challenges and provide flavor solutions engineered for appropriate volatility and exceptional performance.
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.com |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
| 📍 Factory Address | Room 701, Building 3, No. 16, Binzhong South Road, Daojiao Town, Dongguan City, Guangdong Province, China |
Contact us today to elevate your formulations at the molecular level.
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy