Author: R&D Team, CUIGUAI Flavoring
Published by: Guangdong Unique Flavor Co., Ltd.
Last Updated: Jan 30, 2026

Advanced Industrial Chemical Mixing
The e-liquid manufacturing landscape has undergone a seismic shift over the last decade. As consumer preferences move toward high-performance pod systems and high-intensity disposable devices, the demand for “High-Flavor Load” (HFL) formulations—often exceeding 20% or even 30% flavor concentrate by volume—has become the industry standard. However, for the flavor chemist, formulation scientist, and production engineer, this trend introduces a complex physical-chemical challenge: Viscosity Drift.
Viscosity drift refers to the uncontrolled change in a liquid’s resistance to flow over time. In the context of e-liquids, this phenomenon is not merely a cosmetic issue; it is a fundamental failure of product integrity. It can lead to inconsistent wicking, catastrophic leaking, or premature coil failure (dry hits). When dealing with high flavor loads, the interaction between aroma chemicals, carriers, nicotine, and the base solvents becomes exponentially more volatile.
This technical guide provides an exhaustive exploration of the mechanisms of viscosity drift, the molecular interactions at play, and the advanced manufacturing strategies required to stabilize these high-intensity formulations for global markets.
To control viscosity, one must first master the rheological nature of the base solvents. E-liquids are primarily composed of Vegetable Glycerin (VG) and Propylene Glycol (PG). While these are often treated as simple diluents, their behavior in a complex mixture is governed by the laws of fluid dynamics and thermodynamics.
Standard VG/PG mixtures are generally considered Newtonian fluids. This means their viscosity remains constant regardless of the shear rate applied, provided the temperature and pressure are stable. The dynamic viscosity (η) is defined by the ratio of shear stress (τ) to the shear rate (γ˙):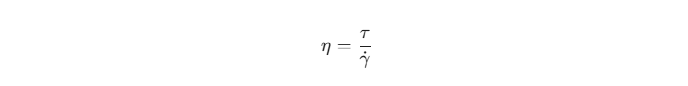
However, when we introduce high concentrations of complex organic molecules (flavorings), the solution can begin to exhibit non-Newtonian tendencies, such as pseudoplasticity (shear-thinning) or even thixotropy (time-dependent thinning under stress).
In a traditional 70/30 VG/PG mix, the introduction of a 25% flavor load (usually PG-based) shifts the actual ratio toward 50/50. This inherent thinning is mathematically predictable. The “drift,” however, refers to the unintended fluctuations that occur after the initial mixing during the product’s shelf life.
In high-flavor load formulations, the sheer volume of organic compounds—esters, ketones, aldehydes, alcohols, and terpenes—creates a “crowded” molecular environment. Several distinct factors contribute to the drift over time.
Both VG and PG are highly hygroscopic, meaning they actively attract and hold water molecules from the surrounding environment. According to the American Chemical Society (ACS), glycerin can absorb significant percentages of its weight in water from the atmosphere depending on ambient humidity.
Water has an extremely low viscosity (approx. 1.0 mPa·s). In a high-flavor load system, the solvent balance is already skewed toward the thinner PG. If even 2–3% water is absorbed due to improper sealing during storage or exposure during high-volume mixing, the total viscosity can drop by as much as 20%. This “atmospheric thinning” is a primary culprit for leaking in pod systems.
Many aroma chemicals act as plasticizers within the VG/PG matrix. For instance, high concentrations of Ethyl Maltol, Vanillin, or certain crystalline coolants (like WS-23) can disrupt the hydrogen bonding network of the glycerin molecules.
As these solids or viscous liquids fully solvate—a process that can take 48 to 120 hours—the internal friction of the liquid decreases. This is the scientific explanation for why a liquid may feel “thicker” immediately after mixing but “thins out” after a few days of steeping. In HFL formulations, where the solute concentration is high, this effect is magnified.
High flavor loads often contain high concentrations of aldehydes (e.g., Cinnamic Aldehyde in cinnamon or Benzaldehyde in cherry/nut flavors). These compounds are prone to oxidation and hydrolysis.
When an ester (a common flavor component) reacts with water (even trace amounts), it can undergo hydrolysis to form an acid and an alcohol: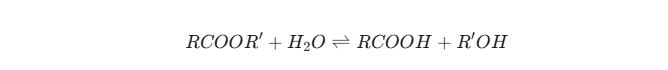
The resulting products often have lower molecular weights and different polarity, which fundamentally alters the fluid’s structural integrity and leads to a decrease in viscosity.
Nicotine is an alkaloid that can act as a catalyst for various chemical reactions. Nicotine salts, formed by the reaction of nicotine with organic acids (like Benzoic, Citric, or Salicilic acid), introduce additional ions into the solution. These ions can interfere with the solvation shells of flavor molecules, leading to unpredictable shifts in the liquid’s rheology over time.
To maintain professional-grade quality control, manufacturers must move beyond simple visual inspections. The use of precision analytical instrumentation is required to quantify drift and ensure batch-to-batch consistency.
The industry standard for measuring e-liquid viscosity is the rotational viscometer (e.g., Brookfield or Anton Paar units). For HFL formulations, it is critical to measure viscosity at multiple temperature points (e.g., 20°C, 25°C, and 45°C) to establish a “Viscosity-Temperature Profile.”
Using the Arrhenius equation, manufacturers can predict long-term viscosity drift by subjecting samples to thermal stress. The rate of chemical reaction (k) increases with temperature: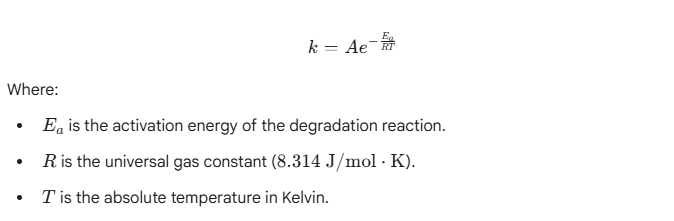
By storing HFL e-liquids at 40°C for 12 weeks, manufacturers can simulate approximately one year of shelf life at room temperature. If the viscosity drops by more than 10% during this period, the formulation is considered unstable.
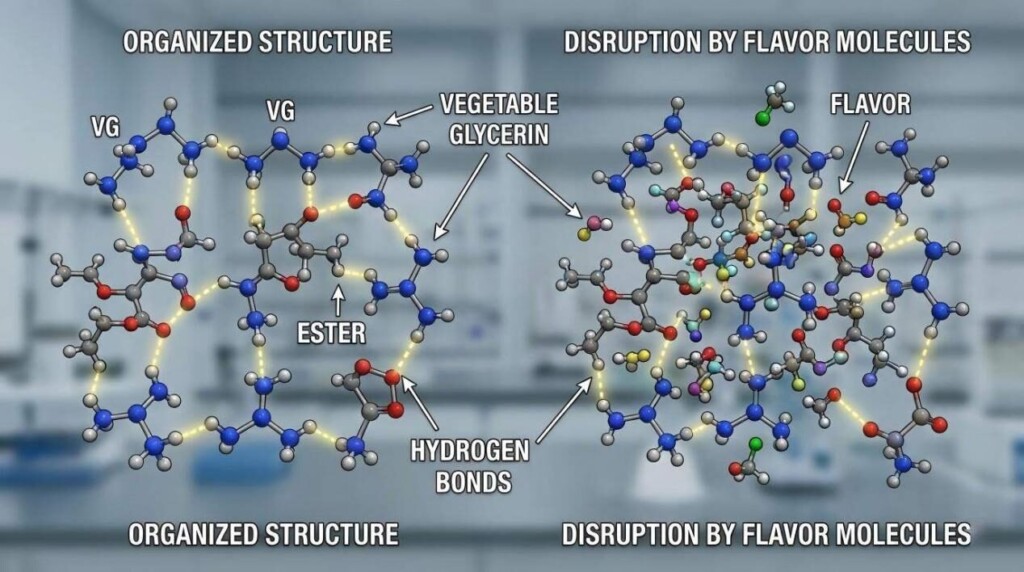
Molecular Interaction of Glycerin and Esters
Controlling viscosity in a 25% or 30% flavor-load formulation requires more than just “adding more VG.” It requires a sophisticated approach to chemical stabilization and co-solvent engineering.
While PG is the default carrier, it is not always the most stable for HFL systems. Forward-thinking manufacturers are exploring alternative carriers:
Traditional paddle or magnetic mixing is often insufficient for high-flavor loads. The “drift” seen in many products is actually the result of the liquid reaching a true equilibrium that wasn’t achieved during a short mix cycle.
The pH of an e-liquid significantly impacts the rate of chemical reactions like esterification and hydrolysis. Most flavorings are slightly acidic. If the formulation becomes too acidic over time, the viscosity will likely drop as the components break down. Utilizing USP-grade buffering agents (such as food-grade sodium citrate) to maintain a pH between 6.2 and 6.8 can effectively “freeze” many of the reactions responsible for drift.
Not all flavors impact viscosity in the same way. According to the Flavor and Extract Manufacturers Association (FEMA), different chemical classes have distinct physical properties that must be accounted for during the formulation phase.
Flavors rich in terpenes (like Limonene in orange or Citral in lemon) are non-polar. When introduced into the polar environment of VG and PG, they act as potent “thinners.” In HFL citrus formulations, the viscosity can drop by as much as 40% compared to a flavorless base. These formulations require a higher initial VG ratio (e.g., 80/20) to settle at a final 70/30 consistency.
Bakery and dessert flavors often rely on high concentrations of Ethyl Maltol, Vanillin, and Acetyl Pyrazine. These are solids at room temperature. When used in high loads, they initially increase viscosity. However, as they interact with the PG/VG over time, they can undergo “re-solvation,” leading to a gradual thinning.
Menthol and synthetic coolants like WS-3 and WS-23 are notorious for their temperature sensitivity. In high concentrations, they can recrystallize if the temperature drops, or cause extreme thinning if the temperature rises. Maintaining a narrow viscosity band in high-coolant liquids requires the use of stabilizers like Distilled Monoglycerides.

Formulation Stability Comparison
To minimize drift, your Standard Operating Procedure (SOP) must be rigorous and scientifically grounded.
High-shear mixing, while effective, introduces micro-bubbles into the liquid. These bubbles can artificially inflate the “apparent viscosity” when measured immediately. Using a vacuum de-aeration chamber or industrial ultrasonic baths post-mix ensures that all air is removed, providing a “true” viscosity reading before the liquid goes to the bottling line.
Oxidation is a primary driver of chemical degradation and subsequent viscosity drift. By purging mixing tanks and storage vessels with Nitrogen, you displace oxygen, effectively halting the oxidative breakdown of aldehydes and nicotine. This is essential for HFL products intended for long-distance export.
Viscosity is highly dependent on temperature. A 5°C difference in the facility can lead to significant variations in fill volume and initial viscosity readings. Standardizing the entire production environment—from mixing to bottling—at a constant 22°C (71.6°F) is a requirement for high-tier manufacturing.
Not all Vegetable Glycerin is created equal. The source (soy, palm, or coconut) and the purity level (USP vs. Food Grade) impact the moisture content. A manufacturer must ensure that their VG has a water content of less than 0.5% to prevent “pre-thinning” of the formulation.
Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) require manufacturers to provide comprehensive stability data as part of PMTA or TPD submissions.
If a product’s viscosity drifts significantly over a 6-month period, regulators may argue that the “Aerosol Delivery” (the amount of vapor and nicotine per puff) has changed. This can lead to the rejection of a marketing application.
A stable viscosity ensures that the liquid wicks into the coil at a constant rate. In a “thin” liquid (low viscosity), the coil may over-saturate, leading to “spit-back” and a higher-than-intended nicotine dose per puff. Conversely, if the liquid is too thick, “dry hits” can occur, producing harmful thermal degradation products like Acrolein and Formaldehyde. Documenting your viscosity control measures is no longer just about quality—it’s about legal and consumer safety.
As we look toward 2026 and beyond, the industry is moving toward “Smart Formulations.” This involves the use of Viscosity Index Improvers (VIIs)—specialized, food-grade cellulose derivatives or specific esters that help maintain a flat viscosity curve across a wider temperature range.
These additives ensure that whether a consumer is vaping in the cold of winter or the heat of summer, the device performs identically. For manufacturers of specialized flavorings, providing “Pre-Stabilized Flavor Bases” that already account for these rheological shifts is the next frontier in B2B service.
| Problem | Potential Cause | Technical Solution |
| Leaking after 2 weeks | Atmospheric water absorption. | Check seal integrity; add 2% Triacetin to formulation. |
| “Thin” taste / Muted flavor | Excessive thinning due to oxidation. | Implement Nitrogen blanketing during mixing. |
| Harshness / Dry hits | Recrystallization of solids (Vanillin/Menthol). | Increase PG ratio or use high-shear homogenization. |
| Inconsistent fill levels | Temperature fluctuations during bottling. | Standardize bottling room temperature to 22°C. |
Controlling viscosity drift in high-flavor load formulations is a multi-disciplinary challenge that sits at the intersection of organic chemistry, fluid dynamics, and industrial engineering. By understanding the hygroscopic nature of your bases, the plasticizing effect of your aroma chemicals, and the necessity of high-shear homogenization, you can produce a product that remains consistent from the first day it is bottled to the last day it is vaped.
In an increasingly competitive market, the winners will be those who prioritize scientific rigor over guesswork. A stable viscosity is the foundation of a premium vaping experience, ensuring flavor clarity, device longevity, and regulatory compliance.
Premium E-Liquid Quality Control
Are you struggling with viscosity inconsistencies in your latest HFL line? Our team of flavor chemists and rheology experts is here to provide the solutions you need to scale with confidence. Whether you require custom-stabilized flavor bases, a full rheological audit of your current line, or help with stability data for regulatory filings, let’s collaborate.
| Contact Channel | Details |
| 🌐 Website: | www.cuiguai.com |
| 📧 Email: | info@cuiguai.com |
| ☎ Phone: | +86 0769 8838 0789 |
| 📱 WhatsApp: | +86 189 2926 7983 |
| 📍 Factory Address | Room 701, Building 3, No. 16, Binzhong South Road, Daojiao Town, Dongguan City, Guangdong Province, China |
The business scope includes licensed projects: food additive production. General projects: sales of food additives; manufacturing of daily chemical products; sales of daily chemical products; technical services, technology development, technical consultation, technology exchange, technology transfer, and technology promotion; biological feed research and development; industrial enzyme preparation research and development; cosmetics wholesale; domestic trading agency; sales of sanitary products and disposable medical supplies; retail of kitchenware, sanitary ware and daily sundries; sales of daily necessities; food sales (only sales of pre-packaged food).
Copyright ©Guangdong Unique Flavor Co., Ltd.All Rights Reserved. Privacy Policy